Immunization Registry Reporting
The objective of this measure is to submit electronic data to health registries.
If you administer immunizations, you can fulfill this measure in Encompass.
- In Encompass on the web, search for the patient.
- Click the Patient Clipboard action bar icon.
- Click the Immunizations tab.
- Click Add Immunization.

- Select or type the immunization information as needed.
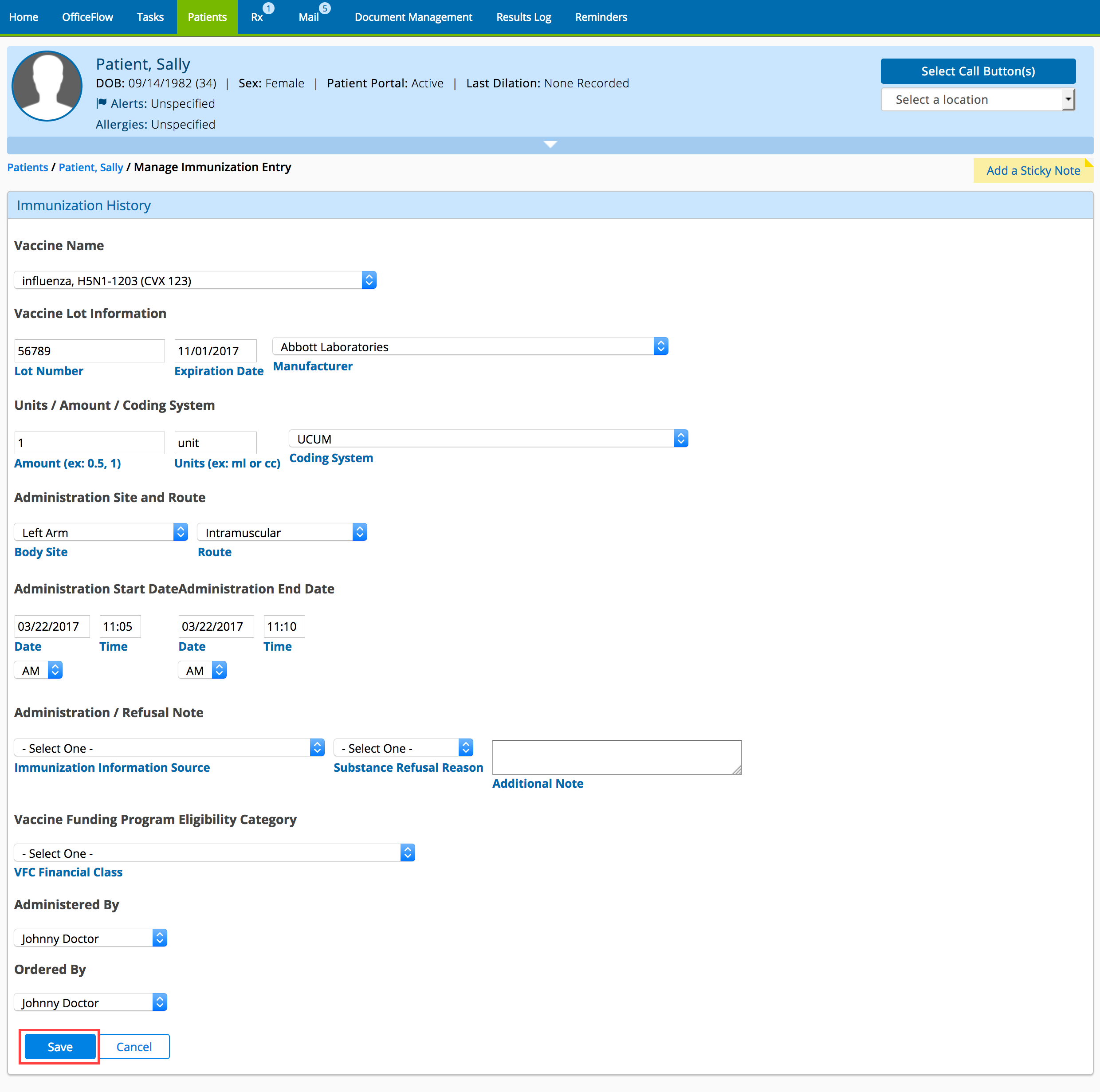
- Click Save.
- Click eSubmit to Immunization Registry.
- Type the registry’s web address for submissions in the Registry URL text box.
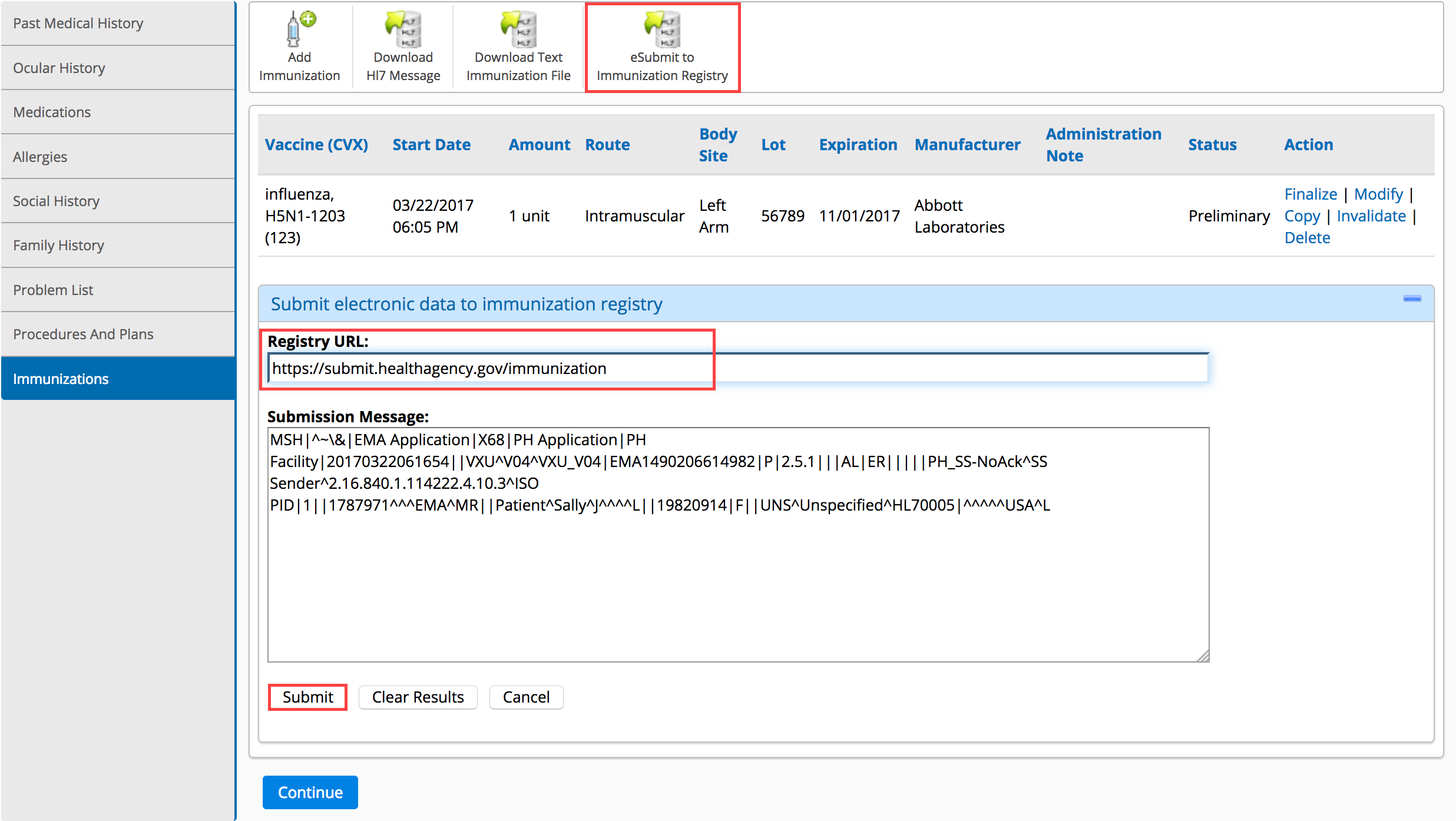
- Click Submit.
The submission results appear below the Submit button. If the submission was successful, continue recording the exam. If the submission failed, correct the errors and try again.
The MIPS-eligible clinician is in active engagement with a public health agency (PHA) to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS).
Active engagement may be demonstrated in one of the following ways:
- Preproduction and Validation. The MIPS-eligible clinician must first register to submit data with the public health agency (PHA) or, where applicable, the clinical data registry (CDR) to which the information is being submitted. Registration must be completed within 60 days after the start of the performance period, while awaiting an invitation from the PHA or CDR to begin testing and validation. MIPS-eligible clinicians that have registered in previous years do not need to submit an additional registration for subsequent performance periods. Upon completion of the initial registration, the MIPS-eligible clinician must begin the process of testing and validation of the electronic submission of data. The MIPS-eligible clinician must respond to requests from the PHA or, where applicable, the CDR within 30 days. Failure to respond twice within a performance period would result in the MIPS-eligible clinician not meeting the measure.
- Validated Data Production. The MIPS-eligible clinician has completed testing and validation of the electronic submission and is electronically submitting production data to the PHA or CDR.
Any MIPS-eligible clinician (EC) who meets one or more of the following criteria may be excluded from the Immunization Registry Reporting measure:
- The EC does not administer any immunizations to any of the populations for which data is collected by its jurisdiction's immunization registry or immunization information system during the performance period; OR
- The EC operates in a jurisdiction for which no immunization registry or immunization information system is capable of accepting the specific standards required to meet the certified EHR definition at the start of the performance period: OR
- The EC operates in a jurisdiction where no immunization registry or immunization information system has declared readiness to receive immunization data as of six months prior to the start of the performance period.
The MIPS eligible clinician must attest YES and report their level of active engagement—preproduction and validation or validated-data production. The Public Health and Clinical Data Exchange objective is worth 25 points.
You must attest YES to both the Immunization Registry Reporting measure and Electronic Case Reporting measure to receive the 25 points for the Public Health and Clinical Data Exchange objective. If you claim an eligible exclusion to both measures, the 25 points for the objective are reallocated to the Provider to Patient Exchange objective.
The following are suggested roles for completing this measure:
- Doctor
- Technician
- Front Desk
If you receive any confirmation for your submission, be it an email or a web page displaying a confirmation message, print it and save it with your other MIPS documentation.