Syndromic Surveillance Data Submission
The objective of this measure is to demonstrate the capability to submit electronic syndromic surveillance data to public health agencies.
- In Eyefinity EHR on the web, create a Visit Note.
- Open the Ocular Exam, record your findings, and Save Visit Note.
If you documented and infection or infectious disease, an HL7 tab appears when you return to the visit note.
- Click the HL7 tab.
- Click the Submit HL7 Message for Public Surveillance.
- Type the registry’s web address for submissions in the Registry URL text box.
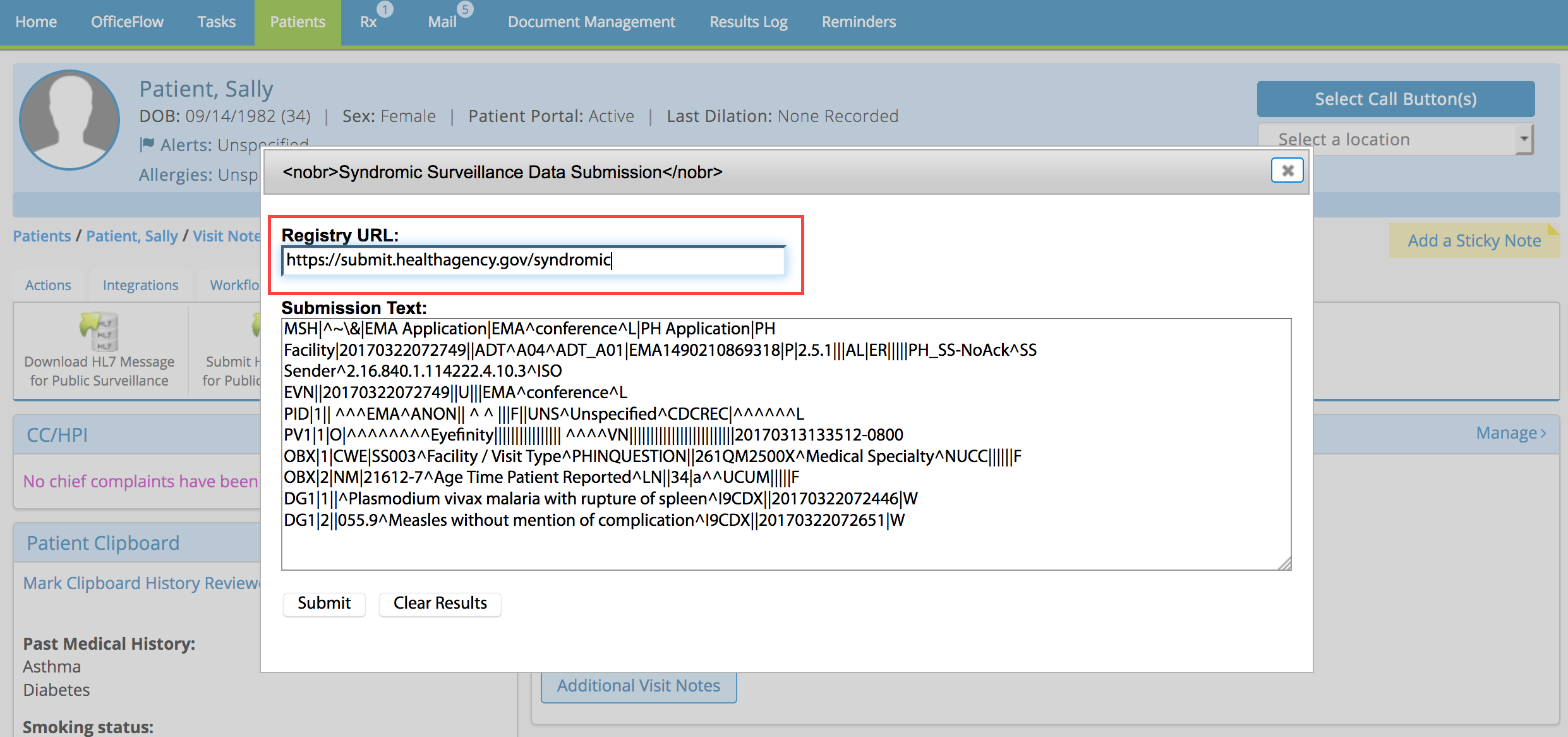
- Click Submit.
The submission results appear below the Submit button. If the submission was successful, continue recording the exam. If the submission failed, correct the errors and try again.
- In Administration, click the Reports toolbar icon.
- Select Public Health Surveillance.
- Record the sending and receiving facility information, receiving application, reporting date range, and ICD-10 code being reported.
- Click Generate HL7 Public Health Files.
- Send the public health files in the DATA\eDocuments\PublicHealth folder to the specified receiving facility. The file names follow the format: ADT_A28_231_Patient ID_date.
The MIPS-eligible clinician is in active engagement with a public health agency to submit syndromic surveillance data from an urgent care setting.
Active engagement can be achieved through any one of the following options:
- Completed Registration to Submit Data. The MIPS-eligible clinician registered to submit data with the PHA to which the information is being submitted; registration was completed within 60 days after the start of the performance period; and the MIPS-eligible clinician is awaiting an invitation from the PHA to begin testing and validation. This option allows MIPS-eligible clinicians to meet the measure when the PHA has limited resources to initiate the testing and validation process. MIPS-eligible clinicians that have registered in previous years do not need to submit an additional registration to meet this requirement for each performance period.
- Testing and Validation. The MIPS-eligible clinician is in the process of testing and validation of the electronic submission of data. MIPS-eligible clinicians must respond to requests from the PHA within 30 days; failure to respond twice within a performance period would result in that MIPS-eligible clinician not meeting the measure.
- Production. The MIPS-eligible clinician has completed testing and validation of the electronic submission and is electronically submitting production data to the PHA.
This measure is not required to fulfill the Public Health and Clinical Data Exchange objective. This measure contributes up to 5 bonus points.
You can receive up to 5 bonus points for one of the following measures. You cannot receive more than 5 bonus points.
- Syndromic Surveillance Data Submission
- Syndromic Surveillance Data Submission
- Clinical Data Registry Reporting
You may receive bonus points even if you claimed an eligible exclusion from both of the required measures and, thus, your Public Health and Clinical Data Exchange objective was reweighted to zero.
The following are suggested roles for completing this measure:
- Doctor
- Technician
- Front Desk
If you receive any confirmation for your submission, be it an email or a web page displaying a confirmation message, print it and save it with your other MIPS documentation.